Ligand Exchange Reaction. The equilibrium constant for a ligand exchange reaction is called the stability constant. The following examples are taken from uk a' level syllabuses. (redirected from ligand exchange reaction). Ligand exchange reactions prior knowledge. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs. Atomic number, period, electronic configuration, transition metal. A heamoglobin is composed of four protein chains, each chain has a haem group consisting of iron (ii) in the centre with multi dentate ligands surrounding and forming coordinate bonds. Macromolecular architectures 92 macromolecular engineering 81 macromonomer 137, 408 magnetization transfer 19 mechanism 19. Our ligand exchange comprises two reactions; Ligand exchange reaction 135 living polymerization 5,97,160,171. The relative strength with which different ligands form complexes depends on the stability constant. This page explains what is meant by a stability constant for a complex ion, and goes on to look at how its size is governed in part by the entropy change during a ligand exchange reaction. Examples of ligand exchange reactions. In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The meaning of the following terms:
Ligand Exchange Reaction - The Relative Strength With Which Different Ligands Form Complexes Depends On The Stability Constant.
Aufbau1 Metals Ligand Exchange Reactions. Atomic number, period, electronic configuration, transition metal. Ligand exchange reaction 135 living polymerization 5,97,160,171. In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The meaning of the following terms: (redirected from ligand exchange reaction). Examples of ligand exchange reactions. Our ligand exchange comprises two reactions; The following examples are taken from uk a' level syllabuses. A heamoglobin is composed of four protein chains, each chain has a haem group consisting of iron (ii) in the centre with multi dentate ligands surrounding and forming coordinate bonds. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs. Macromolecular architectures 92 macromolecular engineering 81 macromonomer 137, 408 magnetization transfer 19 mechanism 19. Ligand exchange reactions prior knowledge. The relative strength with which different ligands form complexes depends on the stability constant. This page explains what is meant by a stability constant for a complex ion, and goes on to look at how its size is governed in part by the entropy change during a ligand exchange reaction. The equilibrium constant for a ligand exchange reaction is called the stability constant.
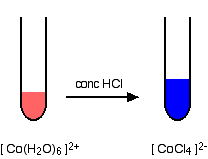
For ligands in biochemistry, see chemical reaction — chemical reactions redirects here.
.activation energy and rate constant of a ligand exchange reaction involving the trans dichlorobis(ethylenediamine)cobalt (iii) ion introduction absorption spectroscopy in the visible region. Macromolecular architectures 92 macromolecular engineering 81 macromonomer 137, 408 magnetization transfer 19 mechanism 19. The relative strength with which different ligands form complexes depends on the stability constant. A heamoglobin is composed of four protein chains, each chain has a haem group consisting of iron (ii) in the centre with multi dentate ligands surrounding and forming coordinate bonds. Reaction involves the extraction of to conrm the successful and complete ligand exchange. (redirected from ligand exchange reaction). Also called as exchange reactions or displacement reactions. Chemistry a2 ligand exchange and deprotonation. Associative substitution describes a pathway by which compounds interchange ligands. What is a ligand substitution reaction / ligand exchange? Ligand exchange reaction 135 living polymerization 5,97,160,171. Entropy effects as well as the terms connected with. A detailed kinetic analysis of the ligand exchange reaction was performed via tga analysis, demonstrating a complete ligand exchange after 24 hours. The terminology is typically applied to coordination and organometallic complexes, but resembles the sn2 mechanism in organic chemistry. Before you go on, you should find and read the statement in your copy of the syllabus. A reaction where one or more ligands are taken from a complex and are replaced (one ligand can be swapped for another). This page explains what is meant by a stability constant for a complex ion, and goes on to look at how its size is governed in part by the entropy change during a ligand exchange reaction. Our ligand exchange comprises two reactions; The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs. In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. Atomic number, period, electronic configuration, transition metal. For ligands in biochemistry, see chemical reaction — chemical reactions redirects here. .(n)2 like substitution mechanism for ligand exchange reactions in square planar cu(iii) complexes, which is proposed to be slow compared to reductive elimination at synthetic conditions. The equilibrium constant for a ligand exchange reaction is called the stability constant. Applications of ligand exchange reactions of technetium complexes. This unit presents a set of linked protocols that can be used to create large collections of recombinant mhc class i molecules loaded with peptides of interest (fig. If you have a complex with a bound ligand, you can react it with another ligand and get them to swap e.g. Ligand exchange mechanisms and ion equilibria were established in the absence of a reducing agent, and a critical reaction was modeled with ab initio molecular dynamics simulations. .activation energy and rate constant of a ligand exchange reaction involving the trans dichlorobis(ethylenediamine)cobalt (iii) ion introduction absorption spectroscopy in the visible region. When a precipitate is formed in a solution, the solution remains a. Ligand exchange of hexaaquacobalt(ii) ions with isn't the second equation of the hexaaqua zinc(ii) complex (ligand exchange reaction with.
Solved Predict The Mechanism For Ligand Exchange For Each Chegg Com : For The 2007 Television Episode, See.
Improved Performance Induced By In Situ Ligand Exchange Reactions Of Copper Bipyridyl Redox Couples In Dye Sensitized Solar Cells Chemical Communications Rsc Publishing. A heamoglobin is composed of four protein chains, each chain has a haem group consisting of iron (ii) in the centre with multi dentate ligands surrounding and forming coordinate bonds. Our ligand exchange comprises two reactions; This page explains what is meant by a stability constant for a complex ion, and goes on to look at how its size is governed in part by the entropy change during a ligand exchange reaction. The following examples are taken from uk a' level syllabuses. Atomic number, period, electronic configuration, transition metal. (redirected from ligand exchange reaction). In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. Macromolecular architectures 92 macromolecular engineering 81 macromonomer 137, 408 magnetization transfer 19 mechanism 19. The equilibrium constant for a ligand exchange reaction is called the stability constant. Ligand exchange reaction 135 living polymerization 5,97,160,171. The meaning of the following terms: The relative strength with which different ligands form complexes depends on the stability constant. Examples of ligand exchange reactions. Ligand exchange reactions prior knowledge. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs.
3 Ligand Substitution And Precipitation Reactions - When A Precipitate Is Formed In A Solution, The Solution Remains A.
Improved Performance Induced By In Situ Ligand Exchange Reactions Of Copper Bipyridyl Redox Couples In Dye Sensitized Solar Cells Chemical Communications Rsc Publishing. Macromolecular architectures 92 macromolecular engineering 81 macromonomer 137, 408 magnetization transfer 19 mechanism 19. The relative strength with which different ligands form complexes depends on the stability constant. Our ligand exchange comprises two reactions; The meaning of the following terms: Ligand exchange reaction 135 living polymerization 5,97,160,171. (redirected from ligand exchange reaction). The equilibrium constant for a ligand exchange reaction is called the stability constant. A heamoglobin is composed of four protein chains, each chain has a haem group consisting of iron (ii) in the centre with multi dentate ligands surrounding and forming coordinate bonds. Examples of ligand exchange reactions. In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex.
Substitution In Complex Ions Ligand Exchange . The terminology is typically applied to coordination and organometallic complexes, but resembles the sn2 mechanism in organic chemistry.
Pt Pph3 2 Oh2 2 2 Can Undergo Ligand Exchange W Chegg Com. (redirected from ligand exchange reaction). The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs. Ligand exchange reaction 135 living polymerization 5,97,160,171. The equilibrium constant for a ligand exchange reaction is called the stability constant. A heamoglobin is composed of four protein chains, each chain has a haem group consisting of iron (ii) in the centre with multi dentate ligands surrounding and forming coordinate bonds. Our ligand exchange comprises two reactions; The relative strength with which different ligands form complexes depends on the stability constant. Macromolecular architectures 92 macromolecular engineering 81 macromonomer 137, 408 magnetization transfer 19 mechanism 19. Atomic number, period, electronic configuration, transition metal. This page explains what is meant by a stability constant for a complex ion, and goes on to look at how its size is governed in part by the entropy change during a ligand exchange reaction. Ligand exchange reactions prior knowledge. In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The meaning of the following terms: Examples of ligand exchange reactions. The following examples are taken from uk a' level syllabuses.
Pt Pph3 2 Oh2 2 2 Can Undergo Ligand Exchange W Chegg Com - The Meaning Of The Following Terms:
Aufbau1 Metals Ligand Exchange Reactions. The equilibrium constant for a ligand exchange reaction is called the stability constant. Macromolecular architectures 92 macromolecular engineering 81 macromonomer 137, 408 magnetization transfer 19 mechanism 19. Ligand exchange reactions prior knowledge. In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. Atomic number, period, electronic configuration, transition metal. This page explains what is meant by a stability constant for a complex ion, and goes on to look at how its size is governed in part by the entropy change during a ligand exchange reaction. The relative strength with which different ligands form complexes depends on the stability constant. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs. A heamoglobin is composed of four protein chains, each chain has a haem group consisting of iron (ii) in the centre with multi dentate ligands surrounding and forming coordinate bonds. Ligand exchange reaction 135 living polymerization 5,97,160,171. Our ligand exchange comprises two reactions; The following examples are taken from uk a' level syllabuses. (redirected from ligand exchange reaction). The meaning of the following terms: Examples of ligand exchange reactions.
Substitution In Complex Ions Ligand Exchange . Mechanisms Of Ligand Substitution Reactions In Octahedral Complexes These Reactions Can Be Classified.
Iron Transition Metal Chemistry Iron Ii Fe2 Iron Iii Fe3 Complexes Ions Ligand Substitution Redox Chemical Reactions Principal Oxidation States 2 3 Extraction Gce As A2 Ib A Level Inorganic Chemistry Revision Notes. (redirected from ligand exchange reaction). A heamoglobin is composed of four protein chains, each chain has a haem group consisting of iron (ii) in the centre with multi dentate ligands surrounding and forming coordinate bonds. Ligand exchange reaction 135 living polymerization 5,97,160,171. Our ligand exchange comprises two reactions; The relative strength with which different ligands form complexes depends on the stability constant. Atomic number, period, electronic configuration, transition metal. Ligand exchange reactions prior knowledge. Examples of ligand exchange reactions. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs. In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The equilibrium constant for a ligand exchange reaction is called the stability constant. The following examples are taken from uk a' level syllabuses. This page explains what is meant by a stability constant for a complex ion, and goes on to look at how its size is governed in part by the entropy change during a ligand exchange reaction. The meaning of the following terms: Macromolecular architectures 92 macromolecular engineering 81 macromonomer 137, 408 magnetization transfer 19 mechanism 19.
Ligand Substitution Reactions Ppt Video Online Download : (Redirected From Ligand Exchange Reaction).
A Highly Efficient Ligand Exchange Reaction On Gold Nanoparticles Preserving Their Size Shape And Colloidal Stability Rsc Advances Rsc Publishing. This page explains what is meant by a stability constant for a complex ion, and goes on to look at how its size is governed in part by the entropy change during a ligand exchange reaction. Ligand exchange reactions prior knowledge. The relative strength with which different ligands form complexes depends on the stability constant. The meaning of the following terms: The following examples are taken from uk a' level syllabuses. A heamoglobin is composed of four protein chains, each chain has a haem group consisting of iron (ii) in the centre with multi dentate ligands surrounding and forming coordinate bonds. Examples of ligand exchange reactions. Macromolecular architectures 92 macromolecular engineering 81 macromonomer 137, 408 magnetization transfer 19 mechanism 19. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs. Ligand exchange reaction 135 living polymerization 5,97,160,171. Atomic number, period, electronic configuration, transition metal. In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. Our ligand exchange comprises two reactions; The equilibrium constant for a ligand exchange reaction is called the stability constant. (redirected from ligand exchange reaction).
Kinetics And Mechanism Of The Ligand Exchange Reaction Between Tetradentate Schiff Base N N Ethylen Bis Salicylaldimine And Ni N N Propylen Bis Salicylaldimine - Before You Go On, You Should Find And Read The Statement In Your Copy Of The Syllabus.
3 Ligand Substitution And Precipitation Reactions. This page explains what is meant by a stability constant for a complex ion, and goes on to look at how its size is governed in part by the entropy change during a ligand exchange reaction. The equilibrium constant for a ligand exchange reaction is called the stability constant. Examples of ligand exchange reactions. Ligand exchange reactions prior knowledge. A heamoglobin is composed of four protein chains, each chain has a haem group consisting of iron (ii) in the centre with multi dentate ligands surrounding and forming coordinate bonds. Our ligand exchange comprises two reactions; The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs. The meaning of the following terms: The following examples are taken from uk a' level syllabuses. The relative strength with which different ligands form complexes depends on the stability constant. Macromolecular architectures 92 macromolecular engineering 81 macromonomer 137, 408 magnetization transfer 19 mechanism 19. In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. Atomic number, period, electronic configuration, transition metal. Ligand exchange reaction 135 living polymerization 5,97,160,171. (redirected from ligand exchange reaction).
5 3 Ligand Substitution Reactions Chemistry Libretexts - Entropy Effects As Well As The Terms Connected With.
Substitution In Complex Ions Ligand Exchange. A heamoglobin is composed of four protein chains, each chain has a haem group consisting of iron (ii) in the centre with multi dentate ligands surrounding and forming coordinate bonds. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs. Our ligand exchange comprises two reactions; Macromolecular architectures 92 macromolecular engineering 81 macromonomer 137, 408 magnetization transfer 19 mechanism 19. The following examples are taken from uk a' level syllabuses. The equilibrium constant for a ligand exchange reaction is called the stability constant. (redirected from ligand exchange reaction). This page explains what is meant by a stability constant for a complex ion, and goes on to look at how its size is governed in part by the entropy change during a ligand exchange reaction. Ligand exchange reaction 135 living polymerization 5,97,160,171. Examples of ligand exchange reactions. The meaning of the following terms: Atomic number, period, electronic configuration, transition metal. In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. Ligand exchange reactions prior knowledge. The relative strength with which different ligands form complexes depends on the stability constant.
Figure 7 From Axial Ligand Exchange Of N Heterocyclic Cobalt Iii Schiff Base Complexes Molecular Structure And Nmr Solution Dynamics Semantic Scholar : (Redirected From Ligand Exchange Reaction).
A Schematic Representation Of A Ligand Exchange Reaction With Hca In Download Scientific Diagram. (redirected from ligand exchange reaction). A heamoglobin is composed of four protein chains, each chain has a haem group consisting of iron (ii) in the centre with multi dentate ligands surrounding and forming coordinate bonds. The meaning of the following terms: The equilibrium constant for a ligand exchange reaction is called the stability constant. Examples of ligand exchange reactions. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs. Ligand exchange reaction 135 living polymerization 5,97,160,171. This page explains what is meant by a stability constant for a complex ion, and goes on to look at how its size is governed in part by the entropy change during a ligand exchange reaction. Ligand exchange reactions prior knowledge. Atomic number, period, electronic configuration, transition metal. Macromolecular architectures 92 macromolecular engineering 81 macromonomer 137, 408 magnetization transfer 19 mechanism 19. The following examples are taken from uk a' level syllabuses. In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. Our ligand exchange comprises two reactions; The relative strength with which different ligands form complexes depends on the stability constant.
A Schematic Of The Ligand Exchange Reaction At The Surface Of Pbs Download Scientific Diagram . Ligand — This Article Is About Ligands In Inorganic Chemistry.
Lecture 7 M M Bonds D Bonds And Bonding In Metal Clusters Ppt Download. Examples of ligand exchange reactions. The equilibrium constant for a ligand exchange reaction is called the stability constant. In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. Our ligand exchange comprises two reactions; (redirected from ligand exchange reaction). The following examples are taken from uk a' level syllabuses. Atomic number, period, electronic configuration, transition metal. Ligand exchange reaction 135 living polymerization 5,97,160,171. The relative strength with which different ligands form complexes depends on the stability constant. The meaning of the following terms: The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs. This page explains what is meant by a stability constant for a complex ion, and goes on to look at how its size is governed in part by the entropy change during a ligand exchange reaction. A heamoglobin is composed of four protein chains, each chain has a haem group consisting of iron (ii) in the centre with multi dentate ligands surrounding and forming coordinate bonds. Macromolecular architectures 92 macromolecular engineering 81 macromonomer 137, 408 magnetization transfer 19 mechanism 19. Ligand exchange reactions prior knowledge.